Shaping the Future of Therapeutics
Poster Session A
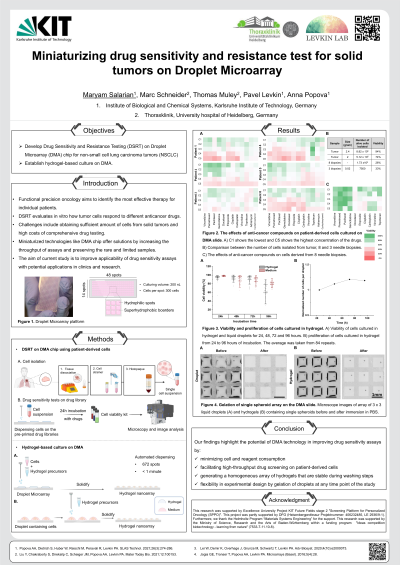
(1010-A) Miniaturizing drug sensitivity and resistance test for solid tumors on Droplet Microarray
Tuesday, May 28, 2024
16:30 - 17:15 CEST
Location: Exhibit Hall

Maryam Salarian, M.Sc.
PhD student
Karlsruhe Institute of Technology
Karlsruhe, Baden-Wurttemberg, Germany
Tony B. Poster Author(s)
Abstract: Functional precision oncology offers a way to narrow down the choices of cancer therapy, by providing treatment strategies for patients based on responses of individual tumors to experimental drug testing. Drug Sensitivity and Resistance Testing (DSRT) involves treating cancer cells from biopsies with various anticancer drugs in vitro to determine the most effective treatment for each patient. There are several challenges associated with this approach, such as obtaining a sufficient number of cells from solid tumors, especially from minimal needle biopsies, and the high costs of comprehensive drug testing with large drug libraries. Miniaturized technologies such as Droplet Microarray (DMA) present promising solutions for these challenges. DMA consists of a microscope glass slide coated with superhydrophobic–hydrophilic patterning. The superhydrophobic coating enables the creation of an array of nanoliter droplets that can be used to culture and test cells.
In this study, we aim to develop DSRT on DMA chip for non-small cell lung carcinoma (NSCLC). We have successfully dissociated six tumors into cell suspensions and cultured the cells in droplets with a volume of 200 nanoliters. These cells were then treated with a library of cytotoxic drugs on a chip. Additionally, we are developing a protocol for isolating cells from minimal needle biopsies. We have demonstrated the initial results from isolating cells from these samples, culturing and testing them with anticancer drugs on chip.
In addition to liquid-media based culture on DMA, we aim to establish a hydrogel-based culture of NSCLC to develop a 3D model that could closer represent the complexity of the tumor microenvironment. Cancer cells exist in a 3D structure within tumors and interact with various cell types and their microenvironment. Therefore, it is important to study cells, particularly primary patient-derived cells, under conditions that closely mimic their in vivo environment. To validate hydrogel-based culture on DMA, we have utilized Dextran-PEG hydrogel and standard cell lines. We successfully generated a homogeneous array of hydrogel on the chip, dispensed in less than 1 minute in a high-throughput manner on 672 spots of the slide. Cells maintained high viability and were able to proliferate. Furthermore, our results confirm the stability of the hydrogels during the washing procedure, facilitating additional biological assays such as staining and cell migration assay.
Our findings highlight the potential of DMA technology in improving drug sensitivity assays, with potential applications in clinics and research settings. By minimizing cell and reagent consumption, DMA facilitates high-throughput drug screening on patient-derived cells. Also, culturing cells in hydrogel provides a more physiologically relevant environment for studying cells.
In this study, we aim to develop DSRT on DMA chip for non-small cell lung carcinoma (NSCLC). We have successfully dissociated six tumors into cell suspensions and cultured the cells in droplets with a volume of 200 nanoliters. These cells were then treated with a library of cytotoxic drugs on a chip. Additionally, we are developing a protocol for isolating cells from minimal needle biopsies. We have demonstrated the initial results from isolating cells from these samples, culturing and testing them with anticancer drugs on chip.
In addition to liquid-media based culture on DMA, we aim to establish a hydrogel-based culture of NSCLC to develop a 3D model that could closer represent the complexity of the tumor microenvironment. Cancer cells exist in a 3D structure within tumors and interact with various cell types and their microenvironment. Therefore, it is important to study cells, particularly primary patient-derived cells, under conditions that closely mimic their in vivo environment. To validate hydrogel-based culture on DMA, we have utilized Dextran-PEG hydrogel and standard cell lines. We successfully generated a homogeneous array of hydrogel on the chip, dispensed in less than 1 minute in a high-throughput manner on 672 spots of the slide. Cells maintained high viability and were able to proliferate. Furthermore, our results confirm the stability of the hydrogels during the washing procedure, facilitating additional biological assays such as staining and cell migration assay.
Our findings highlight the potential of DMA technology in improving drug sensitivity assays, with potential applications in clinics and research settings. By minimizing cell and reagent consumption, DMA facilitates high-throughput drug screening on patient-derived cells. Also, culturing cells in hydrogel provides a more physiologically relevant environment for studying cells.
