Shaping the Future of Therapeutics
Poster Session A
(1008-A) High-throughput drug-repurposing screening identifies FAK as a potential modulator of the suppressive functions of regulatory T cells.
Tuesday, May 28, 2024
16:30 - 17:15 CEST
Location: Exhibit Hall
Nuria García Díaz
Postdoctoral researcher
University of Oslo, Oslo University Hospital
Oslo, Oslo, Norway
Tony B. Poster Author(s)
Abstract: Regulatory T cells (Tregs) play a crucial role in regulating immune responses through suppressing effector T cells (Teffs) activity. In the cancer context, Tregs accumulate in the tumor microenvironment inhibiting anti-tumor immunity and facilitating tumor growth. Multiple efforts have been focused on developing antibodies targeting surface markers in Tregs, but off-target effects have been found due to the overlapping expression profile within other T cell subsets. FoxP3 is the lineage-defining transcription factor of Tregs and regulates the expression of key genes for their suppressive activity. Thus, targeting FoxP3+Tregs could conceivably improve current cancer immunotherapies.
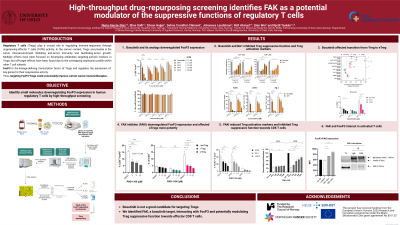
In this study we stablished a high-throughput cell-based drug screening to identify compounds downregulating FoxP3 expression in human primary T cells. CD3+ T cells were isolated and incubated with the compounds in pre-printed 384-well plates overnight, followed by fixation, permeabilization and CD4 and FoxP3 antibody staining using BioMek i7 or Echo550 (Beckman Coulter) automated workstations, and then high-throughput flow cytometry analysis using the iQue screener. Finally, the changes in the percentage of FoxP4 in CD4+ T cells were analysed. The drug library is composed of 1520 FDA/EMA-approved small molecules (Prestwick Chemical Library), so the potential effective compounds could be repurposed as Treg therapy for cancer treatment.
Among other hit compounds, we validated bosutinib, a multi-tyrosine kinase inhibitor, as a FoxP3 down-regulator. To identify similar compounds with more potent effects, we searched for chemical analogs in silico. However, bosutinib analogs failed at reducing FoxP3 expression more potently than the parental compound. Furthermore, bosutinib inhibited Treg-mediated suppression of Teffs proliferation and reduced the expression of surface markers associated with the suppressive function of Tregs. To characterize the mechanisms whereby bosutinib inhibited the suppressive functions of Tregs, we analysed intracellular signalling pathways in stimulated T cells by phospho-flow cytometry. Interestingly, focal adhesion kinase (FAK), a known bosutinib target, was more potently activated and consequently more inhibited in Tregs compared to Teffs upon short T cell receptor (TCR) stimulation. By analysing different Treg subsets, FAK was more activated in resting Tregs (rTregs, low suppressive activity) compared to effector Tregs (eTregs, high suppressive activity) or non-Tregs (proinflammatory profile) upon short TCR stimulation. However, after 2 days of stimulation, eTregs were more bosutinib-sensitive, suggesting a potential role of FAK in the transition of rTregs to a more suppressive status (eTregs). To further study the role of FAK in Treg-mediated immunosuppression, we made use of a specific FAK inhibitor which reduced FoxP3 levels and Treg activity-associated expression of surface markers as well as inhibited CD8 proliferation after co-culturing with pre-treated Tregs.
Although further studies are needed, our high-throughput screening for small molecule inhibitors of FoxP3 expression identified FAK as an important player in the modulation of the immunosuppressive activity of Tregs.
In this study we stablished a high-throughput cell-based drug screening to identify compounds downregulating FoxP3 expression in human primary T cells. CD3+ T cells were isolated and incubated with the compounds in pre-printed 384-well plates overnight, followed by fixation, permeabilization and CD4 and FoxP3 antibody staining using BioMek i7 or Echo550 (Beckman Coulter) automated workstations, and then high-throughput flow cytometry analysis using the iQue screener. Finally, the changes in the percentage of FoxP4 in CD4+ T cells were analysed. The drug library is composed of 1520 FDA/EMA-approved small molecules (Prestwick Chemical Library), so the potential effective compounds could be repurposed as Treg therapy for cancer treatment.
Among other hit compounds, we validated bosutinib, a multi-tyrosine kinase inhibitor, as a FoxP3 down-regulator. To identify similar compounds with more potent effects, we searched for chemical analogs in silico. However, bosutinib analogs failed at reducing FoxP3 expression more potently than the parental compound. Furthermore, bosutinib inhibited Treg-mediated suppression of Teffs proliferation and reduced the expression of surface markers associated with the suppressive function of Tregs. To characterize the mechanisms whereby bosutinib inhibited the suppressive functions of Tregs, we analysed intracellular signalling pathways in stimulated T cells by phospho-flow cytometry. Interestingly, focal adhesion kinase (FAK), a known bosutinib target, was more potently activated and consequently more inhibited in Tregs compared to Teffs upon short T cell receptor (TCR) stimulation. By analysing different Treg subsets, FAK was more activated in resting Tregs (rTregs, low suppressive activity) compared to effector Tregs (eTregs, high suppressive activity) or non-Tregs (proinflammatory profile) upon short TCR stimulation. However, after 2 days of stimulation, eTregs were more bosutinib-sensitive, suggesting a potential role of FAK in the transition of rTregs to a more suppressive status (eTregs). To further study the role of FAK in Treg-mediated immunosuppression, we made use of a specific FAK inhibitor which reduced FoxP3 levels and Treg activity-associated expression of surface markers as well as inhibited CD8 proliferation after co-culturing with pre-treated Tregs.
Although further studies are needed, our high-throughput screening for small molecule inhibitors of FoxP3 expression identified FAK as an important player in the modulation of the immunosuppressive activity of Tregs.
