Screening Applications & Diagnostics
Poster Session A
(1044-A) High-Throughput Screening for Inhibitors of Calcineurin and NFAT Protein-Protein Interaction
Tuesday, May 28, 2024
16:30 - 17:15 CEST
Location: Exhibit Hall
Has Audio
- OM
Ohad Meir, PhD (he/him/his)
Postdoctoral Fellow
Blavatnik Center for Drug Discovery, Tel-Aviv University
Tel Aviv, Tel Aviv, Israel
Poster Presenter(s)
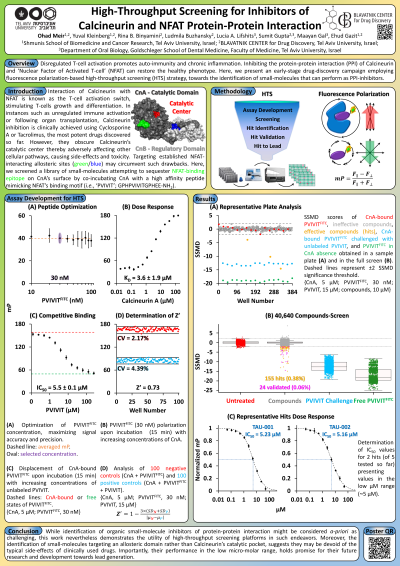
Abstract: T-cell activation is a central component of the immune response. The interaction of the transcription factor ‘Nuclear Factor of Activated T-cell’ (NFAT) with the phosphatase Calcineurin is an important protein-protein interaction (PPI) known as the T-cell activation switch. In its basal state, NFAT is heavily phosphorylated by various kinases, rendering it inactive in the cytoplasm. Rising Ca2+ levels activate Calcineurin, which subsequently dephosphorylates NFAT initiating its nuclear translocation and ensuing transcription of genes such as IL-2, stimulating T-cells growth and differentiation. In some instances, such as unregulated immune activation (often related to chronic inflammations or autoimmune diseases) or following organ transplantation (where immunosuppressants are required), Calcineurin inhibition could restore a balanced T-cell response profile. Indeed, targeting this process has recently been considered as potential strategy to address the notorious cytokine storm often accompanying Covid-19 infections. While Cyclosporine A and Tacrolimus (FK-506) represent the most potent of such drugs discovered so far, they both obscure Calcineurin’s catalytic center thereby adversely affecting several other cellular pathways, resulting in side-effects and inherent toxicity. Thus, the search for drugs inhibiting the Calcineurin-NFAT interaction without obscuring Calcineurin’s catalytic center is ongoing.
Many PPI inhibitors are peptides, rationally designed based on analysis of the proponents binding sites. However, peptides suffer from drawbacks limiting their therapeutic utilization, including protease sensitivity or short half-lives. Therefore, identification of small-molecules that could perform as PPI inhibitors is considered highly attractive, though a priori might be challenging, due to their low surface-area coverage, compared to peptides.
We tackle this challenge by employing a fluorescence-polarization-based high-throughput screening platform, aiming to identify small-molecules capable of inhibiting the interaction between Calcineurin and NFAT. Our assay targets an alternative area on Calcineurin's surface, known to mediate the interaction with NFAT. We co-incubated Calcineurin’s catalytic domain (CnA; containing the binding epitope to NFAT) with a high affinity 14-amino acid peptide mimicking the NFAT binding motif. We quantified the peptide dissociation constant to CnA as kD = 3.6±1.9 µM and established the optimal working conditions of the peptide and CnA respectively as 30 nM and 5 µM, yielding a Z' = 0.73. Having successfully completed a pilot of ~1,300 compounds, we screened >40,000 compounds, with an established hit identification rate of 0.38% and Z' = 0.80±0.07. These hits underwent validation and their IC50 determination is ongoing, towards assessing their potential for chemical optimization, improving their affinity and ADMET properties via medicinal chemistry modifications.
While this research will hopefully produce some attractive hits, it also serves as an example of the advantages of utilizing high-throughput screening platforms in drug discovery, particularly in protein-protein interactions.
Many PPI inhibitors are peptides, rationally designed based on analysis of the proponents binding sites. However, peptides suffer from drawbacks limiting their therapeutic utilization, including protease sensitivity or short half-lives. Therefore, identification of small-molecules that could perform as PPI inhibitors is considered highly attractive, though a priori might be challenging, due to their low surface-area coverage, compared to peptides.
We tackle this challenge by employing a fluorescence-polarization-based high-throughput screening platform, aiming to identify small-molecules capable of inhibiting the interaction between Calcineurin and NFAT. Our assay targets an alternative area on Calcineurin's surface, known to mediate the interaction with NFAT. We co-incubated Calcineurin’s catalytic domain (CnA; containing the binding epitope to NFAT) with a high affinity 14-amino acid peptide mimicking the NFAT binding motif. We quantified the peptide dissociation constant to CnA as kD = 3.6±1.9 µM and established the optimal working conditions of the peptide and CnA respectively as 30 nM and 5 µM, yielding a Z' = 0.73. Having successfully completed a pilot of ~1,300 compounds, we screened >40,000 compounds, with an established hit identification rate of 0.38% and Z' = 0.80±0.07. These hits underwent validation and their IC50 determination is ongoing, towards assessing their potential for chemical optimization, improving their affinity and ADMET properties via medicinal chemistry modifications.
While this research will hopefully produce some attractive hits, it also serves as an example of the advantages of utilizing high-throughput screening platforms in drug discovery, particularly in protein-protein interactions.
