Frontiers in Technology
Poster Session B
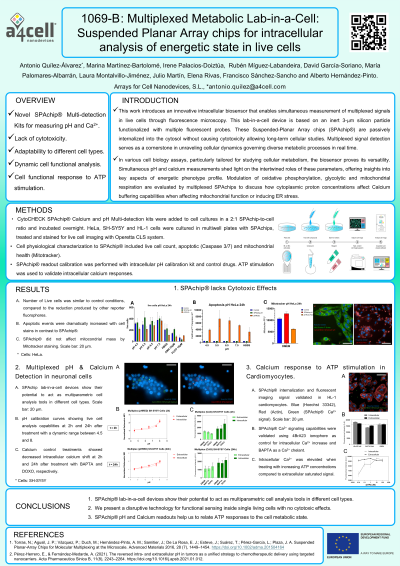
(1069-B) Multiplexed Metabolic Lab-in-a-Cell: Suspended Planar Array chips for intracellular analysis of energetic state in live cells.
Wednesday, May 29, 2024
10:30 - 11:15 CEST
Location: Exhibit Hall

Antonio Quílez-Álvarez, PhD
Head of Applications
A4Cell
Pozuelo De Alarcón, Madrid, Spain
Poster Presenter(s)
Abstract: This work introduces an innovative intracellular biosensor that enables simultaneous measurement of multiplexed signals in live cells through fluorescence microscopy. This lab-in-a-cell device is based on an inert 3-um silicon particle functionalized with multiple fluorescent probes. These Suspended-Planar Array chips (SPAchip®) are passively internalized into the cytosol without causing cytotoxicity allowing long-term cellular studies. Multiplexed signal detection serves as a cornerstone in unraveling cellular dynamics, providing a nuanced understanding of interconnected regulatory networks governing diverse metabolic processes in real time.
Our cutting-edge biosensor, engineered with precision, offers high spatiotemporal resolution, allowing researchers to explore the complex interplay between pH and calcium dynamics within live cells. We emphasize the critical importance of intracellular multiplexed signal detection, allowing kinetic analysis of cytoplasmic molecular crosstalk in diverse signaling pathways.
In various cell biology assays, particularly tailored for studying cellular metabolism, the biosensor proves its versatility. Simultaneous pH and calcium measurements shed light on the intertwined roles of these parameters, offering insights into key aspects of energetic phenotype profile. Modulation of oxidative phosphorylation, glycolytic and mitochondrial respiration are evaluated by multiplexed SPAchips to discuss how cytoplasmic proton concentrations affect Calcium buffering capabilities when affecting mitochondrial function or inducing ER stress.
SPAchip lab-in-a-cell devices show their potential to act as multiparametric cell analysis tools in different cell types, ranging from adherent tumor cells to complex heterogeneous 3D cellular models. These metabolic readouts help us to relate ATP production and cell proliferation capabilities.
We present a disruptive technology for functional sensing inside single living cells, which will enable cell painting strategies to complement their static structural and morphological signatures with dynamic physiological insights.
Altogether, these results highlight a novel approach to real-time intracellular analysis of metabolic energetic status that can help researchers in their drug discovery campaigns, participate in ADME/PK testing or to monitor drug-efficacy or resistance in multiple therapeutic areas. Our work, not only introduces a groundbreaking biosensor but also emphasizes its transformative applications in cell biology assays focused on cell metabolism.
Our cutting-edge biosensor, engineered with precision, offers high spatiotemporal resolution, allowing researchers to explore the complex interplay between pH and calcium dynamics within live cells. We emphasize the critical importance of intracellular multiplexed signal detection, allowing kinetic analysis of cytoplasmic molecular crosstalk in diverse signaling pathways.
In various cell biology assays, particularly tailored for studying cellular metabolism, the biosensor proves its versatility. Simultaneous pH and calcium measurements shed light on the intertwined roles of these parameters, offering insights into key aspects of energetic phenotype profile. Modulation of oxidative phosphorylation, glycolytic and mitochondrial respiration are evaluated by multiplexed SPAchips to discuss how cytoplasmic proton concentrations affect Calcium buffering capabilities when affecting mitochondrial function or inducing ER stress.
SPAchip lab-in-a-cell devices show their potential to act as multiparametric cell analysis tools in different cell types, ranging from adherent tumor cells to complex heterogeneous 3D cellular models. These metabolic readouts help us to relate ATP production and cell proliferation capabilities.
We present a disruptive technology for functional sensing inside single living cells, which will enable cell painting strategies to complement their static structural and morphological signatures with dynamic physiological insights.
Altogether, these results highlight a novel approach to real-time intracellular analysis of metabolic energetic status that can help researchers in their drug discovery campaigns, participate in ADME/PK testing or to monitor drug-efficacy or resistance in multiple therapeutic areas. Our work, not only introduces a groundbreaking biosensor but also emphasizes its transformative applications in cell biology assays focused on cell metabolism.
