Frontiers in Technology
Poster Session A
(1144-A) Optimizing Vaccine Bioprocess Development: End-to-End Automated Workflow Enhancing Efficiency, Traceability and Standardization
Tuesday, May 28, 2024
16:30 - 17:15 CEST
Location: Exhibit Hall

Lilian Ripoll
Laboratory Automation Specialist
Sanofi
69280 Marcy L'Etoile, Rhone-Alpes, France
Poster Presenter(s)
Abstract: Lilian Ripoll, Claire Leroy, Xavier Teixera, Pauline Cornet, Cédric Charretier, Nolwenn Nougarede, Stéphanie Richard
Sanofi R&D, 1541 Av. Marcel Mérieux, 69280 Marcy-l'Étoile, France
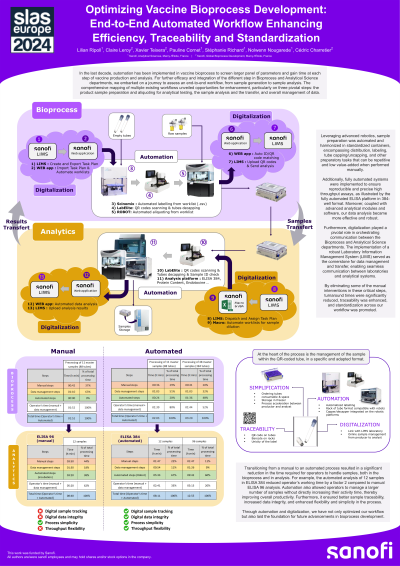
In the last decade, automation has been implemented in vaccine bioprocess to screen larger panel of parameters and gain time at each step of vaccine production and analysis. For further efficacy and integration of the different step in Bioprocess and Analytical Science departments, we embarked on a journey to assess an end-to-end workflow, from sample generation to sample analysis. The comprehensive mapping of multiple existing workflows unveiled opportunities for enhancement, particularly on three pivotal steps: the product sample preparation and aliquoting for analytical testing, the sample analysis, and the transfer and overall management of data.
Leveraging on advanced robotics, sample preparation was automated and harmonized in standardized containers, encompassing distribution, labeling, tube capping/uncapping, and other preparatory tasks that can be repetitive and low value-added when performed manually. Additionally, fully automated systems were implemented to ensure reproducible and precise high throughput assays, as illustrated by the fully automated ELISA platform in 384-well format. Moreover, coupled with advanced analytical modules and software, our data analysis became more effective and robust. Furthermore, digitalization played a pivotal role in orchestrating the communication between Bioprocess and Analytical Science departments. The implementation of a robust Laboratory Information Management System (LIMS) served as the cornerstone for data management and transfer, enabling seamless communication between laboratories and analytical systems. By eliminating some of the manual interventions in these critical steps, turnaround times were significantly reduced, traceability was enhanced, and standardization across our workflow was promoted.
Through automation and digitalization, we have not only optimized our workflow but also laid the foundation for future advancements in bioprocess development.
This work was funded by Sanofi.
LR, CL, XT, PC, CC, NN, and SR are/were Sanofi employees and may hold shares and/or stock options in the company.
Sanofi R&D, 1541 Av. Marcel Mérieux, 69280 Marcy-l'Étoile, France
In the last decade, automation has been implemented in vaccine bioprocess to screen larger panel of parameters and gain time at each step of vaccine production and analysis. For further efficacy and integration of the different step in Bioprocess and Analytical Science departments, we embarked on a journey to assess an end-to-end workflow, from sample generation to sample analysis. The comprehensive mapping of multiple existing workflows unveiled opportunities for enhancement, particularly on three pivotal steps: the product sample preparation and aliquoting for analytical testing, the sample analysis, and the transfer and overall management of data.
Leveraging on advanced robotics, sample preparation was automated and harmonized in standardized containers, encompassing distribution, labeling, tube capping/uncapping, and other preparatory tasks that can be repetitive and low value-added when performed manually. Additionally, fully automated systems were implemented to ensure reproducible and precise high throughput assays, as illustrated by the fully automated ELISA platform in 384-well format. Moreover, coupled with advanced analytical modules and software, our data analysis became more effective and robust. Furthermore, digitalization played a pivotal role in orchestrating the communication between Bioprocess and Analytical Science departments. The implementation of a robust Laboratory Information Management System (LIMS) served as the cornerstone for data management and transfer, enabling seamless communication between laboratories and analytical systems. By eliminating some of the manual interventions in these critical steps, turnaround times were significantly reduced, traceability was enhanced, and standardization across our workflow was promoted.
Through automation and digitalization, we have not only optimized our workflow but also laid the foundation for future advancements in bioprocess development.
This work was funded by Sanofi.
LR, CL, XT, PC, CC, NN, and SR are/were Sanofi employees and may hold shares and/or stock options in the company.
